In this article we provide an overview of the 2023 National Institutes of Health (NIH) Data Management and Sharing (DMS) Policy.
How Did the Policy Change?
In January 2023, the NIH implemented a new Data Management and Sharing Policy. Here is a summary of what changed:
|
Outdated NIH DMS Policy (before 2023) |
2023 NIH DMS Policy |
|
|
Tip: Researchers are no longer required to submit separate Genomic Data Sharing (GDS) Plans. Other sharing policies, such as the model organism sharing policy, remain in effect. NIH has created a tool to help you decide which policies apply to your research.
What data is covered under the 2023 NIH DMS Policy?
The DMS Policy only applies to scientific data, defined by the NIH as data commonly accepted in the scientific community as of sufficient quality to validate and replicate research findings, regardless of whether the data are used to support scholarly publications.
This definition excludes certain types of NIH grants, including:
- Training (Ts)
- Fellowships (Fs)
- Certain non-research Career Awards (e.g., KM1)
- Construction (C06)
- Conference Grants (R13)
- Resources (Gs)
- Research-Related Infrastructure Programs (e.g., S06)
Tip: Be sure to check out the complete list of NIH activity codes subject to the DMS Policy as well as your funding opportunity to determine if the DMS Policy applies to your application.
Under the NIH definition, scientific data do not include:
- Data not necessary for or of sufficient quality to validate and replicate research findings
- Laboratory notebooks
- Preliminary analyses
- Completed case report forms
- Drafts of scientific papers
- Plans for future research
- Peer reviews
- Communications with colleagues
- Physical objects (e.g., laboratory specimens)
Timing
When must data be shared under the new policy? No later than the time of the publication of findings in a peer-reviewed journal or at the end of the award period, whichever comes first.
What to include in your Data Management and Sharing Plan
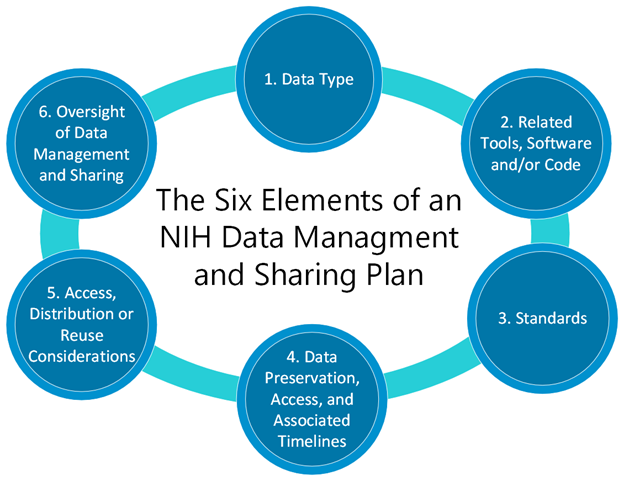
The DMS Plan requires six elements:
- Element 1: Data Type: Identifying data to be preserved and shared
- Element 2: Related Tools, Software, and Code: Tools and software needed to access and manipulate data
- Element 3: Data Standards: Standards to be applied to scientific data and metadata
- Element 4: Data Preservation, Access, and Associated Timelines: Repository to be used, persistent unique identifier, and when and how long data will be made available
- Element 5: Access, Distribution, or Reuse Considerations: Description of factors for data access, distribution, or reuse
- Element 6: Oversight of Data Management and Sharing: How plan compliance will be monitored/managed and by whom
Using the DMPTool
There is currently no specific DMS Plan format required by the NIH. However, we recommend that researchers use DMPTool, a free online wizard that walks you through the process of writing an NIH-compliant DMS Plan.
The NIH recommends, but does not require, that plans be two pages or less.
Application Process
A new “Other Plan(s)” field has been added to the PHS 398 form to collect a single PDF attachment. Data Sharing Plans and Genomic Data Sharing Plans will no longer be submitted to the “Resource Sharing Plan(s)” field.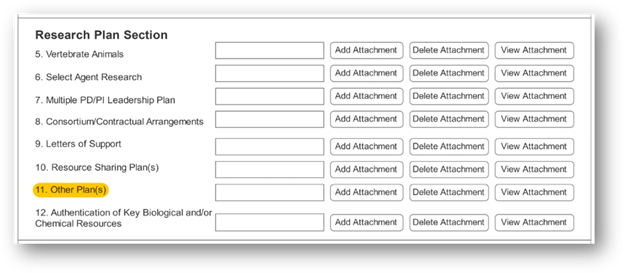
You will also submit a separate budget justification section for any costs related to the DMS Plan. See Article: Budgeting: Estimating Costs for Data Management and Sharing Plans.
Assessment and Enforcement
After submission, a DMS Plan will be assessed by NIH program staff. The program staff will review the plan to see that the six elements have been addressed in a reasonable manner and will determine whether the plan appropriately considers factors that may limit sharing. Peer reviewers will have access to the budget and justification for the DMS Plan to make comments, but the plan will not be scored. The plan can be revised in the Just In Time process to bring it in line with the policy.
After the grant is awarded, the DMS Plan is incorporated into the terms and conditions of the award and must be complied with. You will report the progress of your plan in the Research Performance Progress Report, and the NIH will review your compliance annually. Failure to comply may result in an enforcement action and may affect future funding decisions.
NIH Guide Notice: As outlined in the NIH Policy for Data Management and Sharing, DMS Plans should address six elements (areas): Data Type; Tools, Software, and Code; Data Standards; Data Preservation, Access, and Associated Timelines; Access, Distribution, or Reuse Considerations; and Oversight of Data Management and Sharing, as described in the Application Guide. The NIH suggests that a DMS Plan be no more than two pages. The plan should be attached to the application as a PDF file, as outlined in the NIH’s Format Attachments page.
Additional Resources
- Gonzales S, Carson MB, Holmes K (2022) Ten simple rules for maximizing the recommendations of the NIH data management and sharing plan. PLOS Computational Biology 18(8): e1010397. https://doi.org/10.1371/journal.pcbi.1010397
This article provides 10 key recommendations for creating a DMS Plan that is both maximally compliant and effective. - Data Management and Sharing Plan Format (NIH)
DMSP format from the NIH. Researchers are not required to use this format. - Data Terms related to the NIH DMS Plan and Policy
- Data Management and Sharing Plan Checklist for Researchers
- Extended Reference for DMSP Checklist
- Sharing.nih.gov: Official site for NIH Data Sharing
Related Article
- Sample NIH Data Management and Sharing Plans: Examples of completed DMS Plans.
Sources
National Institutes of Health. “Implementing the NIH Data Management and Sharing (DMS) Policy.” June 2023. https://sharing.nih.gov/sites/default/files/flmngr/DMS-policy-slides-08142023.pptx.
Bohman, Lena. “LibGuides: SOM NIH Data Management and Sharing Policy,” September 2023. https://libguides.hofstra.edu/c.php?g=1275561&p=9358324.
